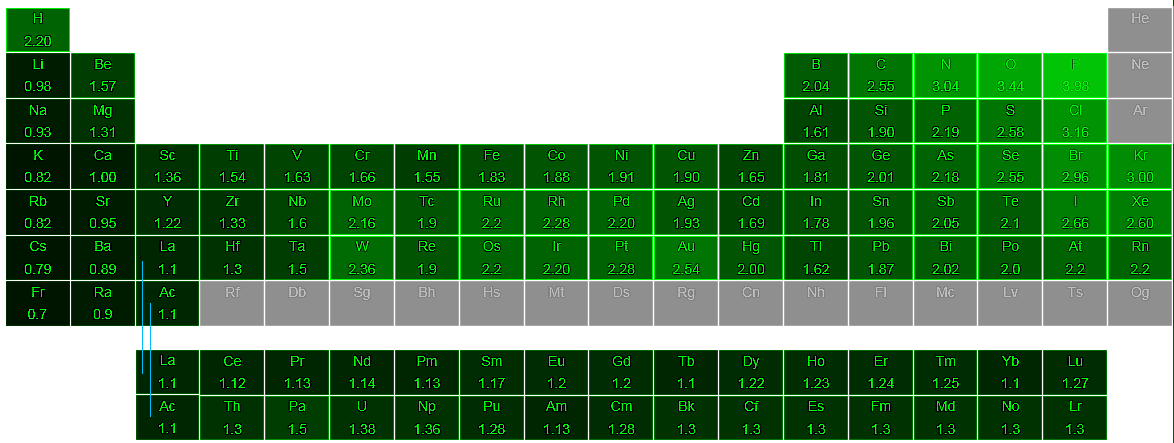
List of elements ordered by electronegativity is listed in the table below with atomic number, chemical symbol and electronegativity value. To list the elements order by electronegativity, click on the table headers. You can print the list of elements by hitting the print button below.
- Electronegativity Definition
- The Most Electronegative Element Is
- Electronegativity Chart
- Electronegativity Of Cf4
What does electronegative mean? Having a negative electric charge. Electronegativity definition at Dictionary.com, a free online dictionary with pronunciation, synonyms and translation. Electronegativity is an atom's tendency to attract electrons to itself in a chemical bond. The most electronegative element is fluorine. The least electronegative or most electropositive element is francium. The greater the difference between atom electronegativity values, the more polar the chemical bond formed between them. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is given a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7.
The element which has the highest electronegativity value is Fluorine with 3.98 χ. And the element which has the lowest electronegativity value is Francium in 0.7 χ.
| Atomic Number | Chemical Symbol | Element Name | Electronegativity χ |
|---|---|---|---|
| 9 | F | Fluorine | 3.98 |
| 8 | O | Oxygen | 3.44 |
| 17 | Cl | Chlorine | 3.16 |
| 7 | N | Nitrogen | 3.04 |
| 36 | Kr | Krypton | 3 |
| 35 | Br | Bromine | 2.96 |
| 53 | I | Iodine | 2.66 |
| 54 | Xe | Xenon | 2.6 |
| 16 | S | Sulfur | 2.58 |
| 6 | C | Carbon | 2.55 |
| 34 | Se | Selenium | 2.55 |
| 79 | Au | Gold | 2.54 |
| 74 | W | Tungsten | 2.36 |
| 82 | Pb | Lead | 2.33 |
| 78 | Pt | Platinum | 2.28 |
| 45 | Rh | Rhodium | 2.28 |
| 44 | Ru | Ruthenium | 2.2 |
| 46 | Pd | Palladium | 2.2 |
| 76 | Os | Osmium | 2.2 |
| 85 | At | Astatine | 2.2 |
| 77 | Ir | Iridium | 2.2 |
| 1 | H | Hydrogen | 2.2 |
| 15 | P | Phosphorus | 2.19 |
| 33 | As | Arsenic | 2.18 |
| 42 | Mo | Molybdenum | 2.16 |
| 52 | Te | Tellurium | 2.1 |
| 51 | Sb | Antimony | 2.05 |
| 5 | B | Boron | 2.04 |
| 83 | Bi | Bismuth | 2.02 |
| 32 | Ge | Germanium | 2.01 |
| 84 | Po | Polonium | 2 |
| 80 | Hg | Mercury | 2 |
| 50 | Sn | Tin | 1.96 |
| 47 | Ag | Silver | 1.93 |
| 27 | Co | Cobalt | 1.91 |
| 75 | Re | Rhenium | 1.9 |
| 14 | Si | Silicon | 1.9 |
| 43 | Tc | Technetium | 1.9 |
| 29 | Cu | Copper | 1.9 |
| 28 | Ni | Nickel | 1.88 |
| 26 | Fe | Iron | 1.83 |
| 31 | Ga | Gallium | 1.81 |
| 49 | In | Indium | 1.78 |
| 48 | Cd | Cadmium | 1.69 |
| 24 | Cr | Chromium | 1.66 |
| 30 | Zn | Zinc | 1.65 |
| 23 | V | Vanadium | 1.63 |
| 81 | Tl | Thallium | 1.62 |
| 13 | Al | Aluminium | 1.61 |
| 41 | Nb | Niobium | 1.6 |
| 4 | Be | Beryllium | 1.57 |
| 25 | Mn | Manganese | 1.55 |
| 22 | Ti | Titanium | 1.54 |
| 91 | Pa | Protactinium | 1.5 |
| 73 | Ta | Tantalum | 1.5 |
| 92 | U | Uranium | 1.38 |
| 93 | Np | Neptunium | 1.36 |
| 21 | Sc | Scandium | 1.36 |
| 40 | Zr | Zirconium | 1.33 |
| 12 | Mg | Magnesium | 1.31 |
| 72 | Hf | Hafnium | 1.3 |
| 99 | Es | Einsteinium | 1.3 |
| 100 | Fm | Fermium | 1.3 |
| 98 | Cf | Californium | 1.3 |
| 101 | Md | Mendelevium | 1.3 |
| 102 | No | Nobelium | 1.3 |
| 103 | Lr | Lawrencium | 1.3 |
| 97 | Bk | Berkelium | 1.3 |
| 96 | Cm | Curium | 1.3 |
| 95 | Am | Americium | 1.3 |
| 90 | Th | Thorium | 1.3 |
| 94 | Pu | Plutonium | 1.28 |
| 71 | Lu | Lutetium | 1.27 |
| 69 | Tm | Thulium | 1.25 |
| 68 | Er | Erbium | 1.24 |
| 67 | Ho | Holmium | 1.23 |
| 66 | Dy | Dysprosium | 1.22 |
| 39 | Y | Yttrium | 1.22 |
| 64 | Gd | Gadolinium | 1.2 |
| 62 | Sm | Samarium | 1.17 |
| 60 | Nd | Neodymium | 1.14 |
| 59 | Pr | Praseodymium | 1.13 |
| 58 | Ce | Cerium | 1.12 |
| 57 | La | Lanthanum | 1.1 |
| 89 | Ac | Actinium | 1.1 |
| 20 | Ca | Calcium | 1 |
| 3 | Li | Lithium | 0.98 |
| 38 | Sr | Strontium | 0.95 |
| 11 | Na | Sodium | 0.93 |
| 88 | Ra | Radium | 0.9 |
| 56 | Ba | Barium | 0.89 |
| 19 | K | Potassium | 0.82 |
| 37 | Rb | Rubidium | 0.82 |
| 55 | Cs | Caesium | 0.79 |
| 87 | Fr | Francium | 0.7 |
- Electronegativity Chart
- Vanadium Electron Configuration
- Boron Electron Configuration
- Strontium Electron Configuration
Electronegativity Definition

The Most Electronegative Element Is
Electronegativity Chart
Electronegativity Of Cf4
