- Apr 20, 2021 valence electrons are those in the The Correct Answer is. Outermost shell. Reason Explained. Outermost shell is correct for valence electrons are those in the.
- Play this game to review Periodic Table. How many valence electrons: 1s2 2s 2 2p6 3s2 3p6 4s 2 3d10 4p3.
- Valence Electrons Are Important Because
- Cached
- Valence Electrons Are Shared
- List Of Valence Electrons For Each Element
- Calculate Number Density Valence Electrons
The key difference between valence and core electrons is that valence electrons participate in chemical bond formations while core electrons do not.
An electron that is found in the outermost energy level of an atom, and that determines the atom's chemical properties. Lewis structure A structural formula in which electrons are represented by dots; dot pairs or dashes between two atomic symbols represent pairs in covalent bonds.
Atoms are the building blocks of all existing substances. They are so tiny that we can’t even observe them with our naked eye. Generally, atoms are in the Angstrom range. Atom is made up of a nucleus, which has protons and neutrons. There are electrons circling around the nucleus in orbitals. Most of the space in an atom is empty. The attractive forces between the positively charged nucleus (positive charge due to protons) and the negatively charged electrons maintain the atom’s shape. Electrons reside in orbitals as pairs in atoms, and they have opposite spins. Moreover, there are two types of electrons as valence electrons and core electrons.
CONTENTS
1. Overview and Key Difference
2. What are Valence Electrons
3. What are Core Electrons
4. Side by Side Comparison – Valence vs Core Electrons in Tabular Form
5. Summary
What are Valence Electrons?
Valence electrons are the electrons in an atom that participate in the chemical bond formation. When chemicals bonds form, an atom can either gain electrons, donate electrons, or share electrons. The ability to donate, gain, or share these electrons depends on the number of valence electrons they have. For example, when an H2 molecule is formed, one hydrogen atom gives one electron to the covalent bond. Thus, two atoms share two electrons. Therefore, a hydrogen atom has one valence electron. In the formation of sodium chloride, one sodium atom gives out one electron, whereas a chlorine atom takes an electron. It happens in order to fill an octet in their valence orbitals. There, sodium has only one valence electron, and chlorine has seven. Therefore, by looking at the valence electrons, we can determine the chemical reactivity of the atoms.
Main group elements (group I, II, III, etc..) have valence electrons in the outermost shells. The number of valence electrons is equivalent to their group number. Inert atoms have completed shells with the maximum number of valence electrons. For transition metals, some inner electrons also act as valence electrons. The number of valence electrons can be determined by looking at the electron configuration of the atom. For instance, nitrogen has the electron configuration of 1s2 2s2 2p3. The electrons in the 2nd shell (which is the highest principal quantum number in this case) are taken as valence electrons. Therefore, nitrogen has five valence electrons. In addition to participating in bonding, valence electrons are the reason for thermal and electrical conductivity of elements.
What are Core Electrons?

Core electrons are the electrons other than valence electrons of the atom. Since these electrons reside at inner locations of the atom, the core electrons do not participate in bond formation. They reside in inner shells of an atom. For example, in a nitrogen atom (1s2 2s2 2p3), five electrons out of all seven are valence electrons, whereas two 1s electrons are core electrons.
Figure 02: Nitrogen has Two Core Electrons
Moreover, the energy required to remove a core electron from an atom is extremely higher than the energy required for valence electrons.
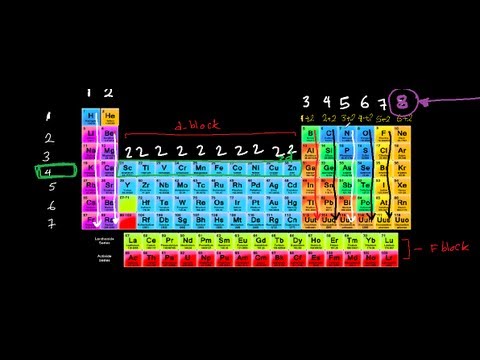
What is the Difference Between Valence and Core Electrons?
Both valence electrons and core electrons move around the nucleus of an atom. Valence electrons reside at the outermost electron shells while core electrons reside at the inner shells. For example, a nitrogen atom has 5 valence electrons and 2 core electrons according to the electron configuration; 1s2 2s2 2p3. Aboveall, the key difference between valence and core electrons is that valence electrons participate in the chemical bond formation, but core electrons do not.
Valence Electrons Are Important Because
Moreover, another significant difference between valence and core electrons is that the energy required to remove core electrons is very high when compared to the energy required to remove valence electrons.
Summary – Valence vs Core Electrons
There are two types of electrons in an atom as valence electrons and core electrons. Valence electrons reside at the outermost shells while core electrons are in the inner shells. The key difference between valence and core electrons is that valence electrons participate in the chemical bond formation while core electrons do not.
Reference:
1. “1.3: Valence Electrons and Open Valences.” Chemistry LibreTexts, Libretexts, 23 Apr. 2019, Available here.
2. “1.9B: Valence and Core Electrons.” Chemistry LibreTexts, Libretexts, 2 May 2019, Available here.
Cached
Image Courtesy:
Valence Electrons Are Shared
1. “Electron shell 011 sodium” By Greg Robson – Application: Inkscape (CC BY-SA 2.0 uk) via Commons Wikimedia
2. “Electron shell 007 Nitrogen” Par Pumbaa (original work by Greg Robson) — File:Electron shell 007 nitrogen.png, (CC BY-SA 2.0 uk) via Commons Wikimedia
List Of Valence Electrons For Each Element
Related posts:
Calculate Number Density Valence Electrons
Atoms in everyday life
|
